In chemistry, the mole fraction or molar fraction ( ) is defined as the amount of a constituent (expressed in moles),
) is defined as the amount of a constituent (expressed in moles), 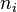 , divided by the total amount of all constituents in a mixture,
, divided by the total amount of all constituents in a mixture,  :
:
 ) is defined as the amount of a constituent (expressed in moles),
) is defined as the amount of a constituent (expressed in moles), 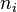 , divided by the total amount of all constituents in a mixture,
, divided by the total amount of all constituents in a mixture,  :
:
The sum of all the mole fractions is equal to 1:
The same concept expressed with a denominator of 100 is the mole percent or molar percentage or molar proportion(mol%).
The mole fraction is also called the amount fraction. It is identical to the number fraction, which is defined as the number of molecules of a constituent  divided by the total number of all molecules
divided by the total number of all molecules  . The mole fraction is sometimes denoted by the lowercase Greek letter
. The mole fraction is sometimes denoted by the lowercase Greek letter 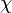 (chi) instead of a Roman
(chi) instead of a Roman 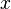 . For mixtures of gases, IUPAC recommends the letter
. For mixtures of gases, IUPAC recommends the letter  .
.
 divided by the total number of all molecules
divided by the total number of all molecules  . The mole fraction is sometimes denoted by the lowercase Greek letter
. The mole fraction is sometimes denoted by the lowercase Greek letter 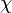 (chi) instead of a Roman
(chi) instead of a Roman 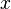 . For mixtures of gases, IUPAC recommends the letter
. For mixtures of gases, IUPAC recommends the letter  .
.
The National Institute of Standards and Technology of the United States prefers the term amount-of-substance fractionover mole fraction because it does not contain the name of the unit mole.
Whereas mole fraction is a ratio of moles to moles, molar concentration is a ratio of moles to volume.
The mole fraction is one way of expressing the composition of a mixture with a dimensionless quantity; mass fraction(percentage by weight, wt%) and volume fraction (percentage by volume, vol%) are others.


This comment has been removed by the author.
ReplyDelete